The Comparative Analysis of Peptides in Enteral Nutrition Products and Foods for Special Medical Purposes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurement of Molecular Weight Distribution of Peptides by High-Performance Liquid Chromatography
2.3. Determination of Short Peptides by UHPLC-OE-MS Analysis
2.4. Peptide Determination by Nanolc-MS/MS Analysis
2.5. Statistical Analysis
3. Results and Discussion
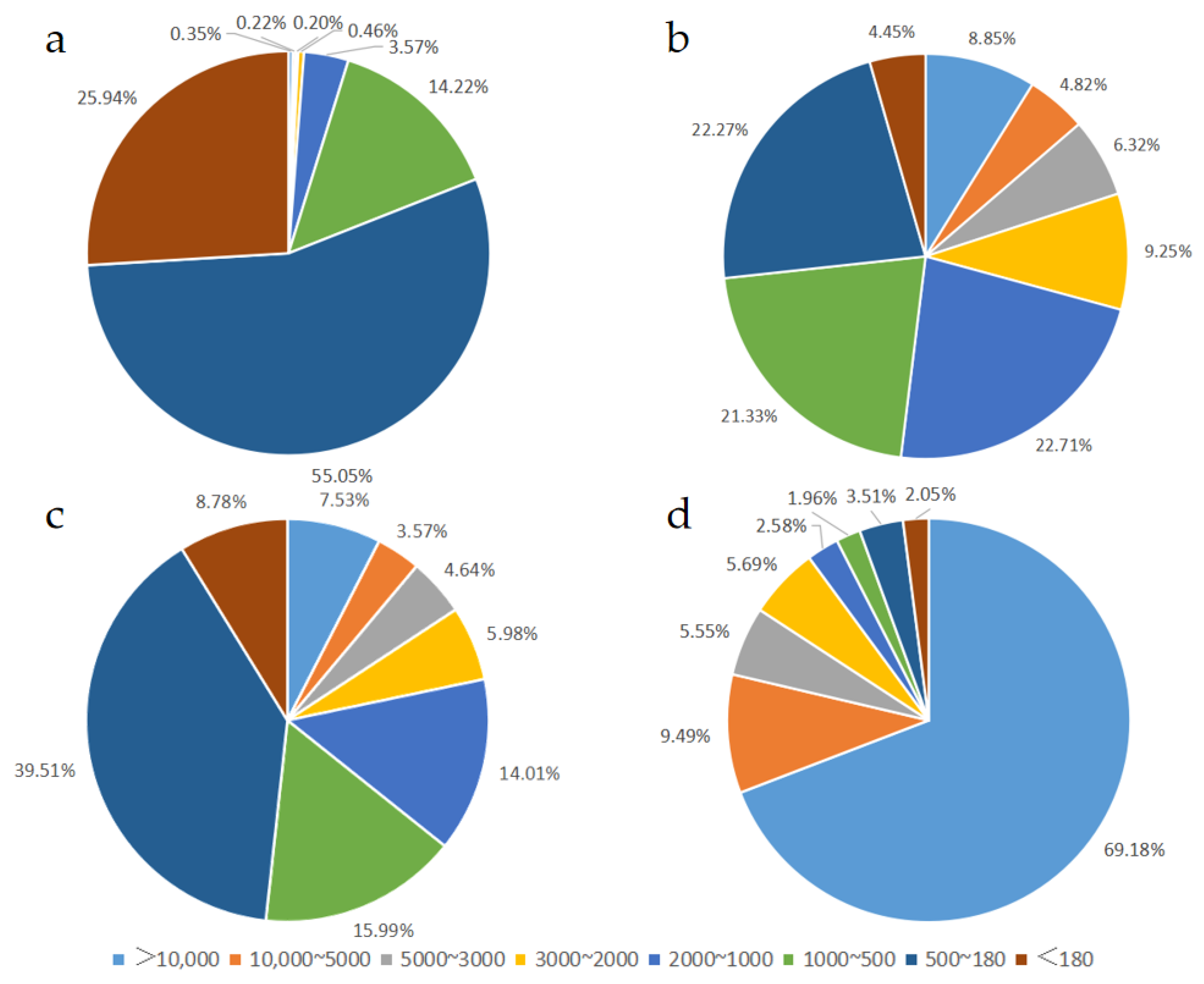
3.1. Molecular Weight Distribution of Peptides in Different EN and FSMP Products
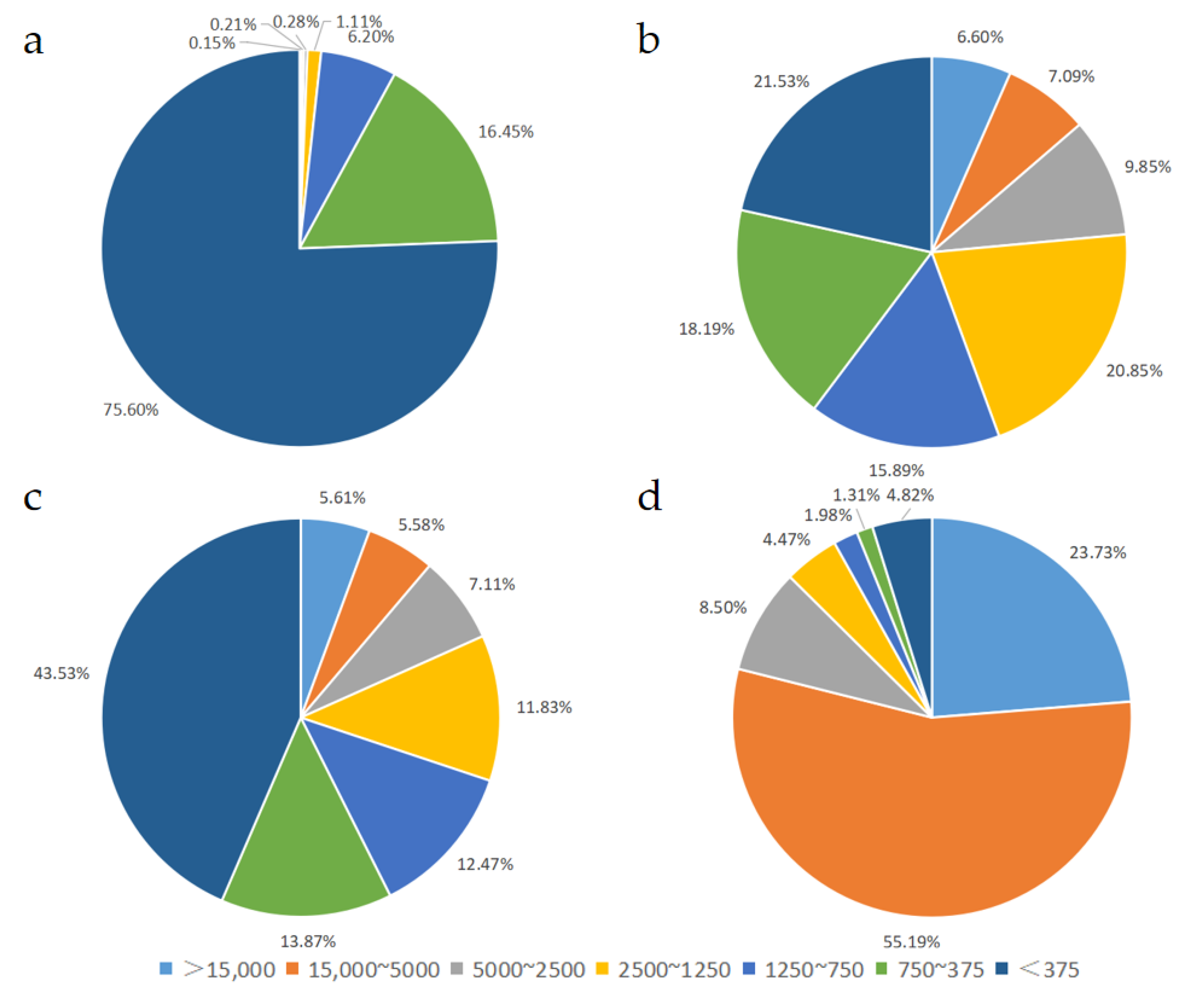
3.2. Short Peptides with Less Than Five Amino Acids in Different EN and FSMP Products
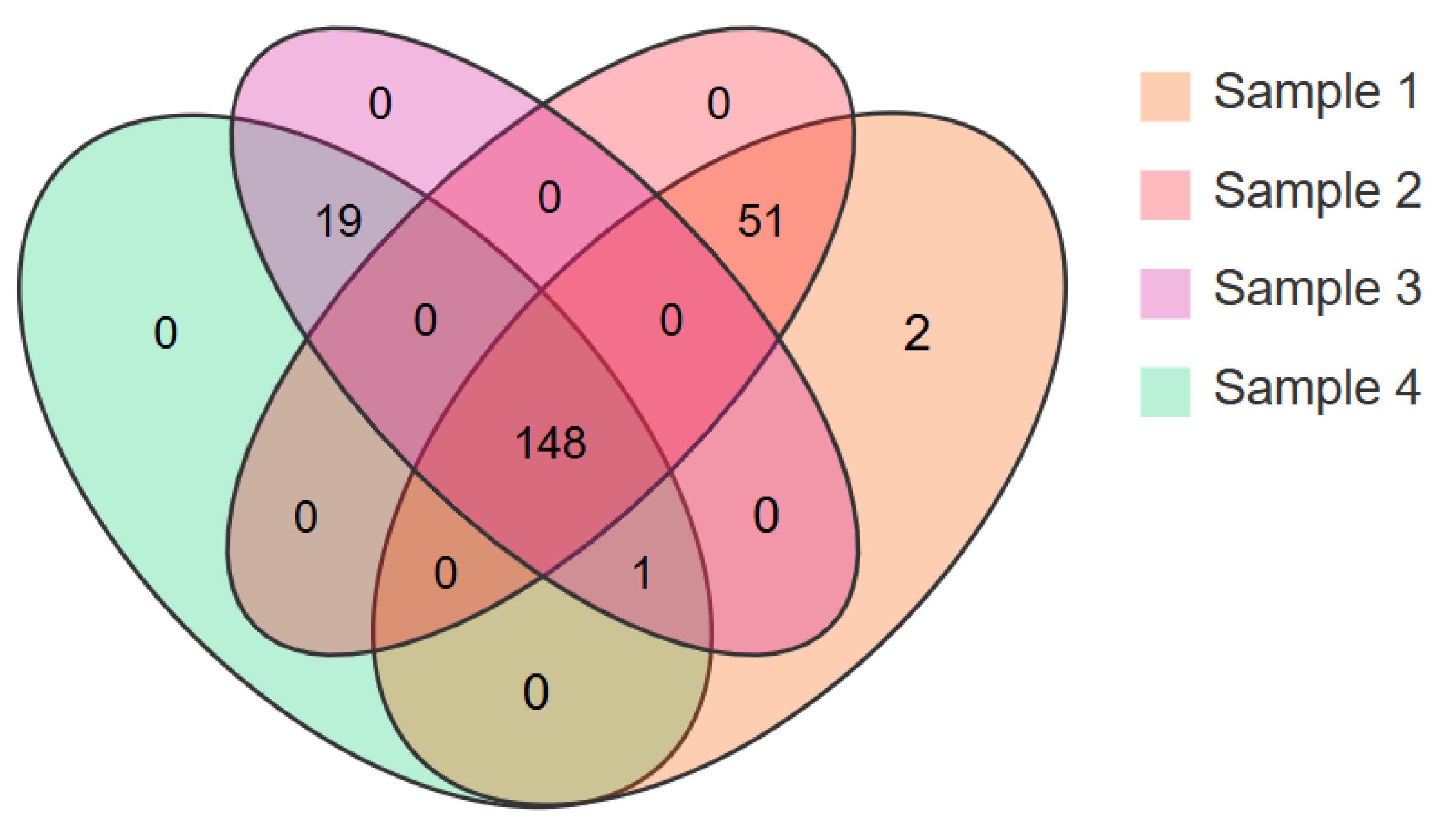
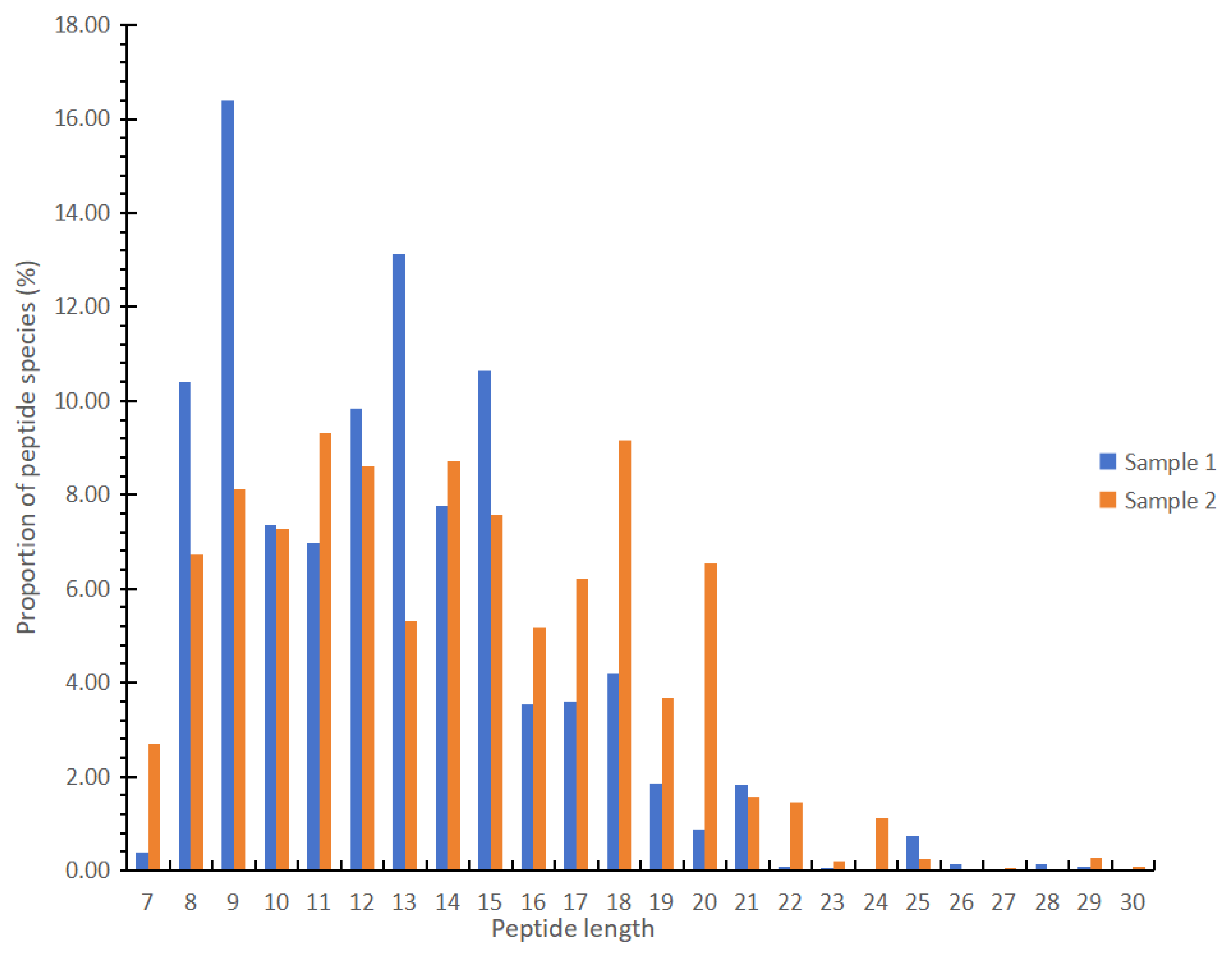
3.3. Peptides with More Than Seven Amino Acids in Different EN Products
3.4. Bioactive Peptides in Different EN and FSMP Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.; Naska, A.; Neuhäuser-Berthold, M.; et al. Scientific and Technical Guidance on Foods for Special Medical Purposes in the Context of Article 3 of Regulation (Eu) No 609/2013. EFSA J. 2021, 19, e06544. [Google Scholar]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. Espen Guidelines on Definitions and Terminology of Clinical Nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, H.Y.; Yan, Y.; Fu, Y.L.; Li, M.F.; Ju, W.H.; Cai, Y.; Liu, Q.; Chen, H.B. Efficacy Observation, Complications and Nursing of Enteral Nutrition Suspension in Patients with Acute Ischemic Stroke. Int. J. Neurosci. 2024, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seron-Arbeloa, C.; Zamora-Elson, M.; Labarta-Monzon, L.; Mallor-Bonet, T. Enteral Nutrition in Critical Care. J. Clin. Med. Res. 2013, 5, 1–11. [Google Scholar] [CrossRef]
- Elfadil, O.M.; Shah, R.N.; Hurt, R.T.; Mundi, M.S. Peptide-Based Formula: Clinical Applications and Benefits. Nutr. Clin. Pract. 2023, 38, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Hong, B.; He, J.J.; Ye, Q.Q.; Hu, Q.Y. Examining the Impact of Early Enteral Nutritional Support on Postoperative Recovery in Patients Undergoing Surgical Treatment for Gastrointestinal Neoplasms. World J. Gastrointest. Surg. 2023, 15, 2222–2233. [Google Scholar] [CrossRef]
- Zhou, Z.W.; Luo, M.D.; Zhang, H.S.; Yin, Y.D.; Cai, Y.P.; Zhu, Z.J. Metabolite Annotation from Knowns to Unknowns through Knowledge-Guided Multi-Layer Metabolic Networking. Nat. Commun. 2022, 13, 6656. [Google Scholar] [CrossRef]
- Rong, L.A.; Fei, Y.J.; Prasad, P.D.; Ramamoorthy, S.; Han, H.; Yang-Feng, T.L.; Hediger, M.A.; Ganapathy, V.; Leibach, F.H. Human Intestinal H+/Peptide Cotransporter-Cloning, Functional Expression, and Chromosomal Localization. J. Biol. Chem. 1995, 270, 6456–6463. [Google Scholar]
- Pavlicevic, M.; Marmiroli, N.; Maestri, E. Immunomodulatory Peptides-a Promising Source for Novel Functional Food Production and Drug Discovery. Peptides 2022, 148, 170696. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, E.Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-Derived Peptidic Antioxidants: A Review of Their Production, Assessment, and Potential Applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Zhu, X.P.; Sun-Waterhouse, D.; Tao, Q.; Li, W.Z.; Shu, D.Y.; Cui, C. The Enhanced Serotonin (5-Ht) Synthesis and Anti-Oxidative Roles of Trp Oligopeptide in Combating Anxious Depression C57BL/6 mice. J. Funct. Foods 2020, 67, 103859. [Google Scholar] [CrossRef]
- Wang, C.; Wang, B.; Li, B. Bioavailability of Peptides from Casein Hydrolysate in Vitro: Amino Acid Compositions of Peptides Affect the Antioxidant Efficacy and Resistance to Intestinal Peptidases. Food Res. Int. 2016, 81, 188–196. [Google Scholar] [CrossRef]
- Chen, M.; Li, B. The Effect of Molecular Weights on the Survivability of Casein-Derived Antioxidant Peptides after the Simulated Gastrointestinal Digestion. Innov. Food Sci. Emerg. Technol. 2012, 16, 341–348. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, W.Q.; Wei, T.; Zhang, C.; Wen, L.; Chen, Q.; Chen, W.; Qiu, J.Y.; Zhang, Y.; Liang, T.B. Effects of Short-Peptide-Based Enteral Nutrition on the Intestinal Microcirculation and Mucosal Barrier in Mice with Severe Acute Pancreatitis. Mol. Nutr. Food Res. 2020, 64, 1901191. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Landa, J.; Calleja-González, J.; León-Guereño, P.; Caballero-García, A.; Córdova, A.; Mielgo-Ayuso, J. Effect of the Combination of Creatine Monohydrate Plus Hmb Supplementation on Sports Performance, Body Composition, Markers of Muscle Damage and Hormone Status: A Systematic Review. Nutrients 2019, 11, 2528. [Google Scholar] [CrossRef] [PubMed]
- Candow, D.G.; Forbes, S.C.; Chilibeck, P.D. Variables Influencing the Effectiveness of Creatine Supplementation as a Therapeutic Intervention for Sarcopenia. Front. Nutr. 2019, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.L.; Matsui, T. Current Knowledge of Intestinal Absorption of Bioactive Peptides. Food Funct. 2017, 8, 4306–4314. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.F.; Zheng, X.Q.; Rui, H.B. Effect of Taurine on Immune Function in Mice with T-Cell Lymphoma During Chemotherapy. Asian Pac. J. Trop. Med. 2017, 10, 1090–1094. [Google Scholar] [CrossRef] [PubMed]
- Schaffer, S.; Kim, H.W. Effects and Mechanisms of Taurine as a Therapeutic Agent. Biomol. Ther. 2018, 26, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Schaffer, S.; Azuma, J. The Effect of Taurine on Chronic Heart Failure: Actions of Taurine against Catecholamine and Angiotensin II. Amino Acids 2014, 46, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Sebih, F.; Mokrane, N.; Fontanel, P.; Kayatekin, M.; Kaabeche, M.; Guiramand, J.; Cohen-Solal, C.; Cens, T.; Rousset, M.; Charnet, P.; et al. The Glutathione Metabolite Γ-Glutamyl-Glutamate Partially Activates Glutamate Nmda Receptors in Central Neurons with Higher Efficacy for Glun2b-Containing Receptors. Front. Pharmacol. 2022, 12, 794680. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Ward, H.E.; Kelly, V.; Palmer, D.; Ankersmit-Udy, A.E.; Lopdell, T.J.; Berry, S.D.; Littlejohn, M.D.; Tiplady, K.; Adams, L.F.; et al. Screening for Phenotypic Outliers Identifies an Unusually Low Concentration of a Β-Lactoglobulin B Protein Isoform in Bovine Milk Caused by a Synonymous Snp. Genet. Sel. Evol. 2022, 54, 22. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Sandström, B.; Jensen, M.; Sørensen, S.S. Casein Phosphopeptides Improve Zinc and Calcium Absorption from Rice-Based but Not from Whole-Grain Infant Cereal. J. Pediatr. Gastroenterol. Nutr. 1997, 24, 56–62. [Google Scholar] [PubMed]
- Capriotti, A.L.; Cavaliere, C.; Piovesana, S.; Samperi, R.; Laganà, A. Recent Trends in the Analysis of Bioactive Peptides in Milk and Dairy Products. Anal. Bioanal. Chem. 2016, 408, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Migliore Samour, D.; Jollès, P. Casein, a Prohormone with an Immunomodulating Role for the Newborn? Experientia 2005, 44, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, H.; Pihlanto, A. Bioactive Peptides: Production and Functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Zaky, A.A.; Simal-Gandara, J.; Eun, J.B.; Shim, J.H.; Abd El-Aty, A.M. Bioactivities, Applications, Safety, and Health Benefits of Bioactive Peptides from Food and by-Products: A Review. Front. Nutr. 2022, 8, 815640. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Wei, Y.; Feng, Z.Y.; Cai, M.Y.; Xu, Y.G.; Gu, R.Z.; Ma, Y.Q.; Pan, X.C. Dipeptides Vl Increase Protein Accumulation in C2c12 Cells by Activating the Akt-Mtor Pathway and Inhibiting the Nf-Κb Pathway. Food Biosci. 2022, 45, 101493. [Google Scholar] [CrossRef]
- Moura, C.S.; Lollo, P.C.B.; Morato, P.N.; Risso, E.M.; Amaya-Farfan, J. Bioactivity of Food Peptides: Biological Response of Rats to Bovine Milk Whey Peptides Following Acute Exercise. Food Nutr. Res. 2017, 61, 1290740. [Google Scholar] [CrossRef] [PubMed]
| Energy Supply Ratio | Product Classification | Product Status | Approval Number | |
|---|---|---|---|---|
| Sample 1 | Protein: 16% Carbohydrate: 69% Fat: 15% | Enteral nutrition suspension (SP) | Liquid | H2***5 |
| Sample 2 | Protein: 18% Carbohydrate: 57% Fat: 25% | Enteral nutrition emulsion (SP) | Liquid | H2***4 |
| Sample 3 | Protein: 14% Carbohydrate: 68% Fat: 18% | Whole Nutritional Formula Food for Special Medical Purposes | Powder | TY2***7 |
| Sample 4 | Protein: 19% Carbohydrate: 47% Fat: 34% | Whole Nutritional Formula Food for Special Medical Purposes | Powder | TY2***5 |
| Relative Molecular Mass Distribution | Retention Time of Each Peak/Min | Peak Area Percentage/% | Number -Average Molecular Weight | Weight -Average Molecular Weight | |
|---|---|---|---|---|---|
| Sample 1 | >10,000 | 12.088 | 0.35 ± 0.61 | 17,810 | 20,482 |
| 10,000~5000 | 13.558 | 0.22 ± 0.35 | 6050 | 6216 | |
| 5000~3000 | 14.589 | 0.20 ± 0.21 | 3631 | 3695 | |
| 3000~2000 | 15.483 | 0.46 ± 0.08 | 2508 | 2729 | |
| 2000~1000 | 16.736 | 3.57 ± 0.14 | 1218 | 1263 | |
| 1000~500 | 17.864 | 14.22 ± 1.01 | 636 | 662 | |
| 500~180 | 19.247 | 55.05 ± 4.85 | 251 | 267 | |
| <180 | 19.800 | 25.94 ± 6.49 | 94 | 113 | |
| Sample 2 | >10,000 | 11.517 | 8.85 ± 2.77 | 16,119 | 17,729 |
| 10,000~5000 | 13.890 | 4.82 ± 0.15 | 6801 | 7091 | |
| 5000~3000 | 14.797 | 6.32 ± 0.08 | 3734 | 3815 | |
| 3000~2000 | 15.515 | 9.25 ± 0.10 | 2387 | 2419 | |
| 2000~1000 | 15.762 | 22.71 ± 1.57 | 1385 | 1442 | |
| 1000~500 | 17.520 | 21.33 ± 0.46 | 694 | 719 | |
| 500~180 | 18.695 | 22.27 ± 3.03 | 313 | 329 | |
| <180 | 20.365 | 4.45 ± 0.91 | 98 | 114 | |
| Sample 3 | >10,000 | 11.740 | 7.53 ± 1.08 | 15,531 | 16,328 |
| 10,000~5000 | 13.890 | 3.57 ± 0.05 | 6788 | 7068 | |
| 5000~3000 | 14.797 | 4.64 ± 0.24 | 3727 | 3807 | |
| 3000~2000 | 15.410 | 5.98 ± 0.18 | 2405 | 2437 | |
| 2000~1000 | 16.714 | 14.01 ± 0.28 | 1342 | 1406 | |
| 1000~500 | 16.745 | 15.99 ± 0.49 | 691 | 722 | |
| 500~180 | 19.108 | 39.51 ± 0.53 | 266 | 281 | |
| <180 | 19.786 | 8.78 ± 1.43 | 86 | 112 | |
| Sample 4 | >10,000 | 12.015 | 69.18 ± 2.08 | 14,543 | 15,454 |
| 10,000~5000 | 12.682 | 9.49 ± 2.46 | 8249 | 8520 | |
| 5000~3000 | 14.620 | 5.55 ± 0.64 | 3498 | 3551 | |
| 3000~2000 | 14.891 | 5.69 ± 0.20 | 2527 | 2558 | |
| 2000~1000 | 15.553 | 2.58 ± 0.22 | 1426 | 1495 | |
| 1000~500 | 16.791 | 1.96 ± 0.08 | 697 | 729 | |
| 500~180 | 19.139 | 3.51 ± 0.11 | 278 | 294 | |
| <180 | 20.454 | 2.05 ± 0.30 | 99 | 109 |
| Relative Molecular Mass Distribution | <180 | 500~180 | 1000~500 | 2000~1000 | 3000~2000 | 5000~3000 | 10,000~5000 | >10,000 |
|---|---|---|---|---|---|---|---|---|
| Sample 1 | 25.94 ± 6.49 a | 55.05 ± 4.85a | 14.22 ± 1.01 c | 3.57 ± 0.14 c | 0.46 ± 0.08 c | 0.20 ± 0.21 d | 0.22 ± 0.35 c | 0.35 ± 0.61 c |
| Sample 2 | 4.45 ± 0.91 bc | 22.27 ± 3.03 c | 21.33 ± 0.46 a | 22.71 ± 1.57 a | 9.25 ± 0.10 a | 6.32 ± 0.08 a | 4.82 ± 0.15 b | 8.85 ± 2.77 b |
| Sample 3 | 8.78 ± 1.43 b | 39.51 ± 0.53 b | 15.99 ± 0.49 b | 14.01 ± 0.28 b | 5.98 ± 0.18 b | 4.64 ± 0.24 c | 3.57 ± 0.05 b | 7.53 ± 1.08 b |
| Sample 4 | 2.05 ± 0.30 c | 3.51 ± 0.11 d | 1.96 ± 0.08 d | 2.58 ± 0.22 c | 5.69 ± 0.20 b | 5.55 ± 0.64 b | 9.49 ± 2.46 a | 69.18 ± 2.08 a |
| Relative Molecular Mass Distribution | Retention Time of Each Peak/Min | Peak Area Percentage/% | Number -Average Molecular Weight | Weight -Average Molecular Weight | |
|---|---|---|---|---|---|
| Sample 1 | >15,000 | 10.857 | 0.15 ± 0.25 | 22,467 | 23,865 |
| 15,000~5000 | 12.876 | 0.21 ± 0.34 | 6904 | 7364 | |
| 5000~2500 | 14.633 | 0.28 ± 0.24 | 4225 | 4345 | |
| 2500~1250 | 15.943 | 1.11 ± 0.84 | 2087 | 2157 | |
| 1250~750 | 16.742 | 6.20 ± 0.68 | 946 | 962 | |
| 750~375 | 17.864 | 16.45 ± 0.76 | 525 | 540 | |
| <375 | 19.247 | 75.60 ± 1.58 | 156 | 201 | |
| Sample 2 | >15,000 | 11.517 | 6.60 ± 0.85 | 18,741 | 19,933 |
| 15,000~5000 | 12.586 | 7.09 ± 2.01 | 7826 | 8570 | |
| 5000~2500 | 15.121 | 9.85 ± 0.16 | 3294 | 3426 | |
| 2500~1250 | 15.762 | 20.85 ± 0.86 | 1712 | 1776 | |
| 1250~750 | 16.560 | 15.89 ± 1.20 | 967 | 989 | |
| 750~375 | 17.520 | 18.19 ± 1.28 | 550 | 571 | |
| <375 | 18.695 | 21.53 ± 3.34 | 209 | 260 | |
| Sample 3 | >15,000 | 11.740 | 5.61 ± 0.61 | 17,107 | 17,681 |
| 15,000~5000 | 12.584 | 5.58 ± 1.69 | 7980 | 8781 | |
| 5000~2500 | 15.121 | 7.11 ± 0.31 | 3310 | 3439 | |
| 2500~1250 | 15.711 | 11.83 ± 0.08 | 1742 | 1807 | |
| 1250~750 | 16.714 | 12.47 ± 0.15 | 968 | 987 | |
| 750~375 | 17.708 | 13.87 ± 0.24 | 537 | 557 | |
| <375 | 19.108 | 43.53 ± 1.14 | 198 | 235 | |
| Sample 4 | >15,000 | 11.797 | 23.73 ± 3.11 | 18,269 | 19,115 |
| 15,000~5000 | 11.863 | 55.19 ± 3.15 | 11,562 | 12,117 | |
| 5000~2500 | 14.513 | 8.50 ± 0.10 | 3152 | 3233 | |
| 2500~1250 | 15.024 | 4.47 ± 0.52 | 1949 | 2019 | |
| 1250~750 | 16.665 | 1.98 ± 0.55 | 908 | 939 | |
| 750~375 | 17.775 | 1.31 ± 0.67 | 500 | 514 | |
| <375 | 19.089 | 4.82 ± 0.53 | 170 | 209 |
| Relative Molecular Mass Distribution | <375 | 750~375 | 1250~750 | 2500~1250 | 5000~2500 | 15,000~5000 | >15,000 |
|---|---|---|---|---|---|---|---|
| Sample 1 | 75.60 ± 1.58 a | 16.45 ± 0.76 b | 6.20 ± 0.68 c | 1.11 ± 0.84 d | 0.28 ± 0.24 d | 0.21 ± 0.34 c | 0.15 ± 0.25 c |
| Sample 2 | 21.53 ± 3.34 c | 18.20 ± 1.28 a | 15.89 ± 1.20 a | 20.85 ± 0.86 a | 9.85 ± 0.16 a | 7.09 ± 2.01 b | 6.60 ± 0.85 b |
| Sample 3 | 43.53 ± 1.14 b | 13.87 ± 0.24 c | 12.47 ± 0.15 b | 11.83 ± 0.08 b | 7.11 ± 0.31 c | 5.58 ± 1.69 b | 5.61 ± 0.61 b |
| Sample 4 | 4.82 ± 0.53 d | 1.31 ± 0.67 d | 1.98 ± 0.55 d | 4.47 ± 0.52 c | 8.50 ± 0.10 b | 55.19 ± 3.15 a | 23.72 ± 3.11 a |
| Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|
| Isoleucine | Leucine | Leucine | Proline |
| Leucine | Arginine | Isoleucine | Leucine |
| Valine | Lysine | Valine | Isoleucine |
| Phenylalanine | Isoleucine | Phenylalanine | Arginine |
| Arginine | Proline | Methionine | Lysine |
| Lysine | Phenylalanine | Taurine | Phenylalanine |
| Proline | Valine | Proline | Histidine |
| Threonine | Tyrosine | Histidine | Valine |
| Homoserine | Asparagine | Threonine | Methionine |
| Methionine | Methionine | Homoserine | Tyrosine |
| Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|
| Leu-Ile | Val-Ile | Val-Phe | Val-Phe |
| Val-Ile | Leu-Ile | Leu-Leu | Phe-Val |
| Leu-Hpro | Val-Tyr | Val-Ile | Val-Ile |
| Val-Phe | Gly-Leu | Leu-Val | Leu-Val |
| Leu-Val | Leu-Phe | Leu-Phe | Leu-Leu |
| Ile-Met | Phe-Leu | Phe-Leu | Leu-Phe |
| Leu-Leu | Tyr-Val | Phe-Val | Phe-Leu |
| Ile-Leu | Leu-Val | Ile-Ala | Val-Tyr |
| Ile-Asp | Pro-Phe | Leu-Ala | Ile-Ala |
| Phe-Val | Thr-Val-Leu | Ile-Met | Ile-Ser |
| Ile-Trp | Ile-Trp | Tyr-Ile | Ile-Met |
| Leu-Ala | Leu-Leu | Ile-Ser | Isoleucyl-Threonine |
| Leu-Glu | Ile-Leu | Gly-Leu | Creatine |
| Ile-Ser | Maculosin | Isoleucyl -Phenylalanine | Ala-Leu |
| Ala-Leu | Val-Phe | Val-Ala | Anorexigenic Peptide |
| Gly-Leu | Thr-Lys | Isoleucyl-Threonine | Leu-Ala |
| Tyr-Ile | Ile-Met | Val-Tyr | Tyr-Val |
| Gly-Gly-Leu | Val-Lys | Thr-Leu | Val-Ala |
| Val-Tyr | Leu-Gly | Gly-Gly-Leu | Ile-Gly-Ile |
| Val-Ala | Ala-Leu | Tyr-Leu | Gly-Leu |
| Protein Descriptions | Peptide Stripped Sequence | Peptide Length | Content Proportion/% | |
|---|---|---|---|---|
| Sample 1 | Beta-casein | YPFPGPIP | 8 | 6.58 |
| Beta-casein | YPFPGPIPN | 9 | 5.98 | |
| Beta-casein | KEMPFPKYP | 9 | 4.89 | |
| Beta-lactoglobulin | KPTPEGDLE | 9 | 3.95 | |
| Beta-casein | APKHKEMPFPKYP | 13 | 3.89 | |
| Beta-casein | KEMPFPKY | 8 | 2.04 | |
| Kappa-casein | AIPPKKNQDKTEIPT | 15 | 1.88 | |
| Zinc-alpha-2 -glycoprotein | SKPREGFPSFQAV | 13 | 1.85 | |
| Zinc-alpha-2 -glycoprotein | PAAQNTKRKWEAEAVYVQ | 18 | 1.68 | |
| Glycosylation -dependent cell adhesion molecule 1 | SRQPQSQNPKLPLSILK | 17 | 1.57 | |
| Beta-lactoglobulin | KGLDIQKVAGTWYSL | 15 | 1.49 | |
| Kappa-casein | IPPKKNQDKTEIPT | 14 | 1.46 | |
| Beta-2-microglobulin | VTLEQPRIVKW | 11 | 1.39 | |
| Beta-2-microglobulin | IQRPPKIQVY | 10 | 1.22 | |
| Beta-2-microglobulin | VTLEQPRIVKWD | 12 | 1.13 | |
| Beta-lactoglobulin | LKPTPEGDLE | 10 | 1.09 | |
| Beta-lactoglobulin | DISLLDAQSAPLRVY | 15 | 1.00 | |
| Xanthine dehydrogenase/oxidase | PTYTSATLLFQKD | 13 | 0.99 | |
| Polymeric immunoglobulin receptor | KSPIFGPEEVTSVEGRSVSIK | 21 | 0.96 | |
| Beta-lactoglobulin | VEELKPTPEGDLEIL | 15 | 0.95 | |
| Sample 2 | Beta-lactoglobulin | VEELKPTPEGDLEILLQK | 18 | 4.93 |
| Beta-lactoglobulin | VYVEELKPTPEGDLEILLQK | 20 | 4.76 | |
| Beta-lactoglobulin | VEELKPTPEGDLEIL | 15 | 2.88 | |
| Beta-lactoglobulin | VYVEELKPTPEGDLEIL | 17 | 2.48 | |
| Beta-lactoglobulin | LVLDTDYKK | 9 | 2.40 | |
| Beta-lactoglobulin | SDISLLDAQSAPLR | 14 | 2.39 | |
| Beta-lactoglobulin | VLVLDTDYKK | 10 | 1.94 | |
| Beta-lactoglobulin | VLDTDYKK | 8 | 1.56 | |
| Beta-lactoglobulin | VLVLDTDYK | 9 | 1.55 | |
| Beta-lactoglobulin | LDTDYKK | 7 | 1.54 | |
| Beta-lactoglobulin | VEELKPTPEGDLEILLQ | 17 | 1.32 | |
| Beta-lactoglobulin | SLLDAQSAPLR | 11 | 1.29 | |
| Beta-lactoglobulin | EVDDEALEKFDK | 12 | 1.29 | |
| Beta-lactoglobulin | SLAMAASDISLLDAQSAPLR | 20 | 1.25 | |
| Beta-lactoglobulin | TPEVDDEALEK | 11 | 1.23 | |
| Beta-lactoglobulin | LIVTQTMK | 8 | 1.22 | |
| Glycosylation -dependent cell adhesion molecule 1 | ILNKPEDETHLEAQPTDASAQF | 22 | 1.21 | |
| Beta-lactoglobulin | TPEVDDEALEKFDK | 14 | 1.21 | |
| Beta-lactoglobulin | VLDTDYK | 7 | 1.15 | |
| Beta-lactoglobulin | SLAMAASDISLL | 12 | 1.01 |
| Sample | Protein Descriptions | Peptide Quantity | Content Proportion/% |
|---|---|---|---|
| Sample 1 | Beta-casein | 14 | 25.89 |
| Beta-lactoglobulin | 71 | 20.49 | |
| Zinc-alpha-2-glycoprotein | 24 | 9.90 | |
| Xanthine dehydrogenase/oxidase | 51 | 9.49 | |
| Glycosylation-dependent cell adhesion molecule 1 | 18 | 6.13 | |
| Beta-2-microglobulin | 5 | 4.13 | |
| Kappa-casein | 7 | 3.89 | |
| Butyrophilin subfamily 1 member A1 | 11 | 1.95 | |
| Sample 2 | Beta-lactoglobulin | 99 | 63.08 |
| Beta-casein | 32 | 7.18 | |
| Glycosylation-dependent cell adhesion molecule 1 | 34 | 6.85 | |
| Alpha-S1-casein | 13 | 4.66 | |
| Albumin | 16 | 2.75 | |
| Alpha-lactalbumin | 7 | 2.16 | |
| Prostaglandin-H2 D-isomerase | 6 | 1.48 | |
| Nucleobindin-1 | 10 | 1.26 |
| Sample 1 | Sample 2 | Sample 3 | Sample 4 |
|---|---|---|---|
| Leu-Ile | Leu-Ile | Val-Phe | Val-Phe |
| Leu-Hpro | Val-Tyr | Leu-Leu | Phe-Val |
| Val-Phe | Gly-Leu | Leu-Val | Leu-Val |
| Leu-Val | Leu-Phe | Leu-Phe | Leu-Leu |
| Ile-Leu | Phe-Leu | Phe-Leu | Leu-Phe |
| Leu-Leu | Tyr-Val | Phe-Val | Phe-Leu |
| Phe-Val | Leu-Val | Leu-Ala | Val-Tyr |
| Ile-Trp | Pro-Phe | Tyr-Ile | Creatine |
| Leu-Ala | Thr-Val-Leu | Gly-Leu | Ala-Leu |
| Ala-Leu | Ile-Trp | Val-Ala | Leu-Ala |
| Gly-Leu | Ile-Leu | Val-Tyr | Tyr-Val |
| Tyr-Ile | Leu-Leu | Tyr-Leu | Val-Ala |
| Val-Tyr | Maculosin | Val-Glu | Gly-Leu |
| Val-Ala | Val-Phe | Thr-Val-Leu | Gly-Pro-Arg-Pro -amide |
| Leu-Phe | Leu-Gly | Tyr-Trp | Tyr-Ile |
| Phe-Leu | Ala-Leu | Pro-Met | Tyr-Trp |
| Thr-Val-Leu | Tyr-Ile | Phe-Met | Leu-Trp |
| Val-Met | Leu-Trp | Tyr-Val | Thr-Val-Leu |
| Tyr-Val | (3xi,6xi)-Cyclo (alanylvalyl) | Ala-Leu | Phe-Met-Arg-Phe -amide |
| Ile-Pro-Ile | Val-Ala | Leu-Asp | Met-Thr |
| Bioactive Peptide Sequence | ||||
|---|---|---|---|---|
| Sample 1 | YPFPGPIP | YPFPGPIPN | LKPTPEGDLE | VEELKPTPEGDLEIL |
| LKPTPEGDLEIL | ELKPTPEGDLEIL | TQTPVVVPPF | LVRTPEVDDE | |
| Sample 2 | VEELKPTPEGDLEIL | VLVLDTDYK | TPEVDDEALEK | VLDTDYK |
| LDAQSAPLR | PFPEVFGK | FFVAPFPEVFGK | ELKPTPEGDLEIL | |
| YLGYLEQLLR | HQPHQPLPPTVMFPPQ | RELEELNVPGEIVESLSSSEESITR | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Fang, C.; Hu, Y.; Xu, J.; Zhao, W.; Li, L. The Comparative Analysis of Peptides in Enteral Nutrition Products and Foods for Special Medical Purposes. Foods 2024, 13, 2557. https://doi.org/10.3390/foods13162557
Li H, Fang C, Hu Y, Xu J, Zhao W, Li L. The Comparative Analysis of Peptides in Enteral Nutrition Products and Foods for Special Medical Purposes. Foods. 2024; 13(16):2557. https://doi.org/10.3390/foods13162557
Chicago/Turabian StyleLi, Hao, Chenlu Fang, Yushan Hu, Jing Xu, Wei Zhao, and Li Li. 2024. "The Comparative Analysis of Peptides in Enteral Nutrition Products and Foods for Special Medical Purposes" Foods 13, no. 16: 2557. https://doi.org/10.3390/foods13162557
APA StyleLi, H., Fang, C., Hu, Y., Xu, J., Zhao, W., & Li, L. (2024). The Comparative Analysis of Peptides in Enteral Nutrition Products and Foods for Special Medical Purposes. Foods, 13(16), 2557. https://doi.org/10.3390/foods13162557







